Tridestra
PRESCRIBING INFORMATION CAN BE FOUND HERE
TRIDESTRA OVERVIEW:
Estradiol valerate is a naturally occuring form of oestrogen (plant origin). Medroxyprogesterone acetate is a synthetic form of progesterone.
Hormone Replacement Therapy for oestrogen deficiency symptoms indicated in peri- and postmenopausal women. Prevention of postmenopausal osteoporosis in women at high risk of future fractures where other therapies are contraindicated or not tolerated.
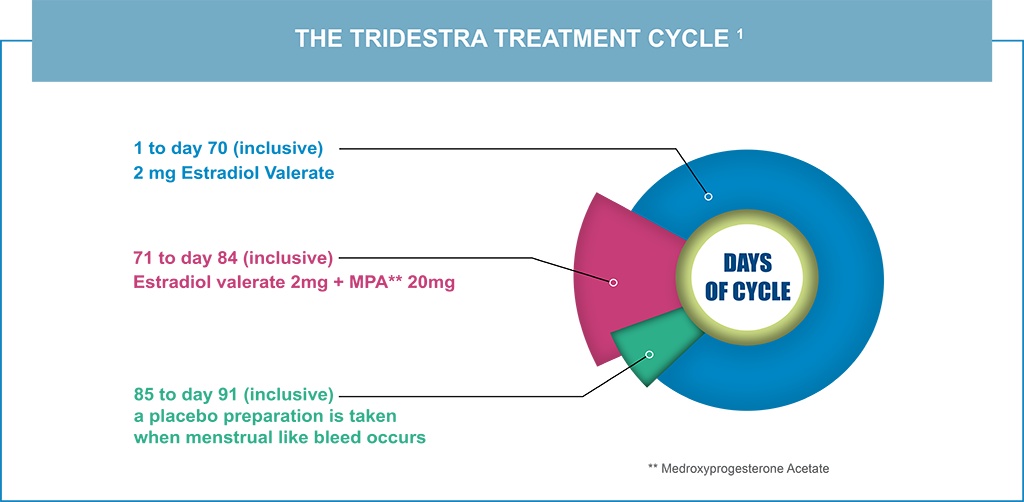
- Offers peri- or postmenopausal women only four periods a year and four cycles of progestogen per year
- Effective relief of climacteric symptoms
- Progestogen offers endometrial protection in women with a uterus
SAFETY PROFILE:
Contraindications
- Known, past or suspected breast cancer
- Known or suspected oestrogen-dependent malignant tumours (e.g. endometrial cancer)
- Undiagnosed genital bleeding
- Untreated endometrial hyperplasia
- Previous idiopathic or current venous thromboembolism
- Known thrombophilic disorders
- Active or recent arterial thromboembolic disease
- Acute liver disease or a history of liver disease
- Hypersensitivity to the active substances
- Porphyria
- Patients with rare hereditary problems of galactose intolerance, total lactase deficiency or glucose-galactose malabsorption should not take this medicine
SIDE EFFECTS:
Adverse drug reactions occur most commonly in the first months of the treatment. They are usually mild and subside with continued treatment.
The most common adverse effect is breakthrough bleeding and spotting appearing in 22% of patients. The overall percentage of treated patients experiencing at least one adverse reaction is 47%.
The common side effects include:
- Increase or decrease of weight
- Depression, nervousness, lethargy
- Headache, dizziness
- Hot flushes
- Nausea, vomiting, stomach cramps, flatulence
- Breasts become tender or painful
- Increased sweating
See SPC for more information.
June 2023/HRT-2bac(1)
| ADVERSE EVENTS SHOULD BE REPORTED. REPORTING FORMS AND INFORMATION CAN BE FOUND AT WWW.MHRA.GOV.UK/YELLOWCARD. ADVERSE EVENTS SHOULD ALSO BE REPORTED TO ORION PHARMA (UK) LTD ON 01635 520300. |
Reference:
- Tridestra Summary of Product Characteristics
